DIY Novel Green Science Potato Digital Clock Educational Kit with 2 inch LCD Screen (Potato NOT Included)(White)
DIY Novel Green Science Potato Digital Clock Educational Kit with 2 inch LCD Screen (Potato NOT Included)(White)
No reviews
Precio habitual
R$ 89,00
Precio habitual
R$ 145,00
Precio de oferta
R$ 89,00
Los gastos de envío se calculan en la pantalla de pago.
Free Shipping Tracked
Estimated delivery: Dec 2 - Dec 12
Cantidad
No se pudo cargar la disponibilidad de retiro
✓ Your Purchase is Protected
Join 40,000+ happy customers who shop risk-free
Free Worldwide Shipping Tracked
Ships in 1-3 days • Save £8.99
6-Month Guarantee Protected
Manufacturing faults covered
30-Day Easy Returns Risk-Free
Changed your mind? Full refund
Secure Checkout SSL
Your payment info is protected
✓ 40,000+ customers trust us with their purchases
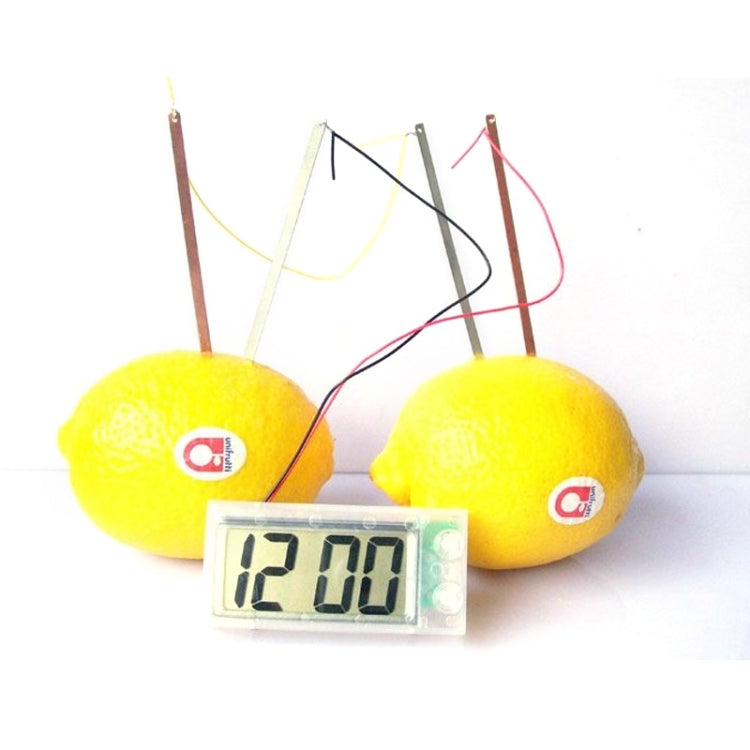
1) You can know how to make a real galvanic battery from potatoes to power a digital clock
2) Zinc and copper electrodes create an electrochemical reaction to generate voltage
3) And converts the chemical energy stored in the metal strips into electrical energy
4) Also learn how to wire up a simple circuit
5) You may experiment with different liquids like salt water, fruit juices or fruit like lemon, orange, tomato besides potato
6) A unique experimental kit that inspires young scientists
7) Mini LCD clock with stand to show the time, month & day
8) With 2 buttons on the clock to adjust the time
9) Environment friendly and lightweight, easy to use
10) Clock size: 66x32x11mm
-Cup size: 62x51x51mm
* How it work
1) The metal strips and potatoes make a simple battery that creates electricity to operate the clock.each potato works as a device called a electrochemical cell. it converts the chemical energy stored in the metal strips into electrical energy .two potatoes are needed to make electricity strong enough to run the clock
2) A cell works because of the chemical properties of the metals inside(in this case the copper and zinc.)the different properties cause tiny particles charged with electricity(called ions)to move between the two strips of metal.this flow is an electric current.the potato contains the particles that allow the current to flow.but it stops the metals touching.electric current also flows along the wires between the zinc and copper strips and the clock.this current makes the clock work.
2) Zinc and copper electrodes create an electrochemical reaction to generate voltage
3) And converts the chemical energy stored in the metal strips into electrical energy
4) Also learn how to wire up a simple circuit
5) You may experiment with different liquids like salt water, fruit juices or fruit like lemon, orange, tomato besides potato
6) A unique experimental kit that inspires young scientists
7) Mini LCD clock with stand to show the time, month & day
8) With 2 buttons on the clock to adjust the time
9) Environment friendly and lightweight, easy to use
10) Clock size: 66x32x11mm
-Cup size: 62x51x51mm
* How it work
1) The metal strips and potatoes make a simple battery that creates electricity to operate the clock.each potato works as a device called a electrochemical cell. it converts the chemical energy stored in the metal strips into electrical energy .two potatoes are needed to make electricity strong enough to run the clock
2) A cell works because of the chemical properties of the metals inside(in this case the copper and zinc.)the different properties cause tiny particles charged with electricity(called ions)to move between the two strips of metal.this flow is an electric current.the potato contains the particles that allow the current to flow.but it stops the metals touching.electric current also flows along the wires between the zinc and copper strips and the clock.this current makes the clock work.
Share